Oxygen(O) electron configuration and orbital diagram (2022)
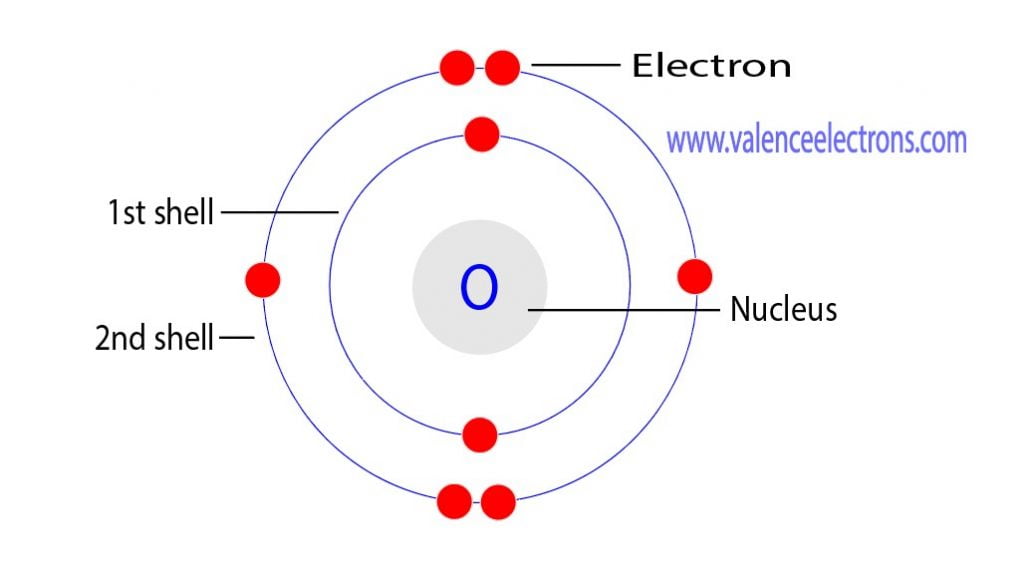
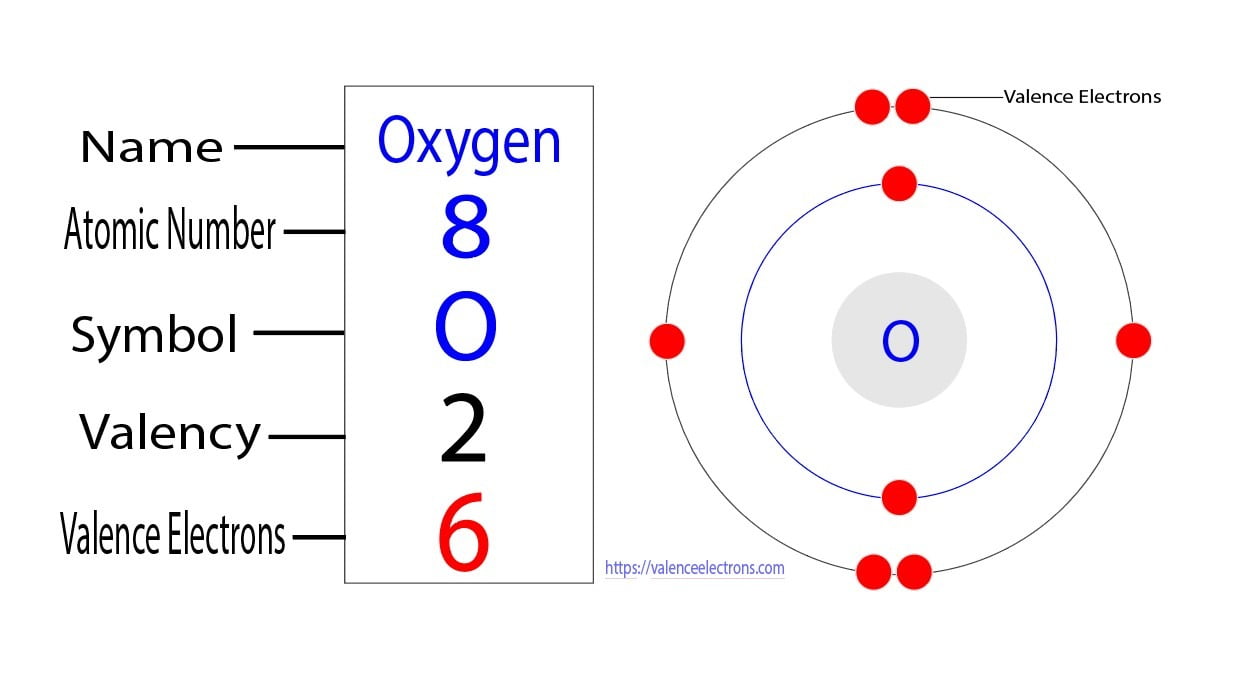
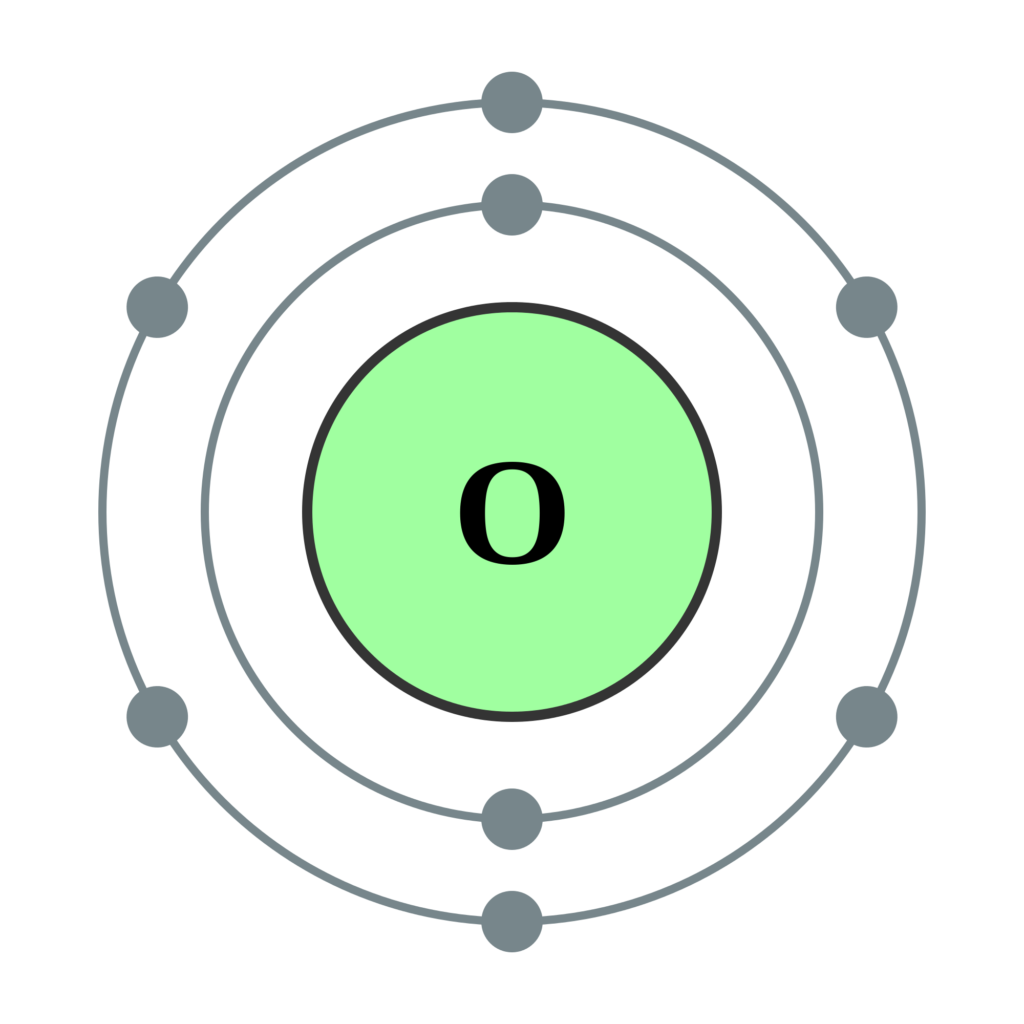
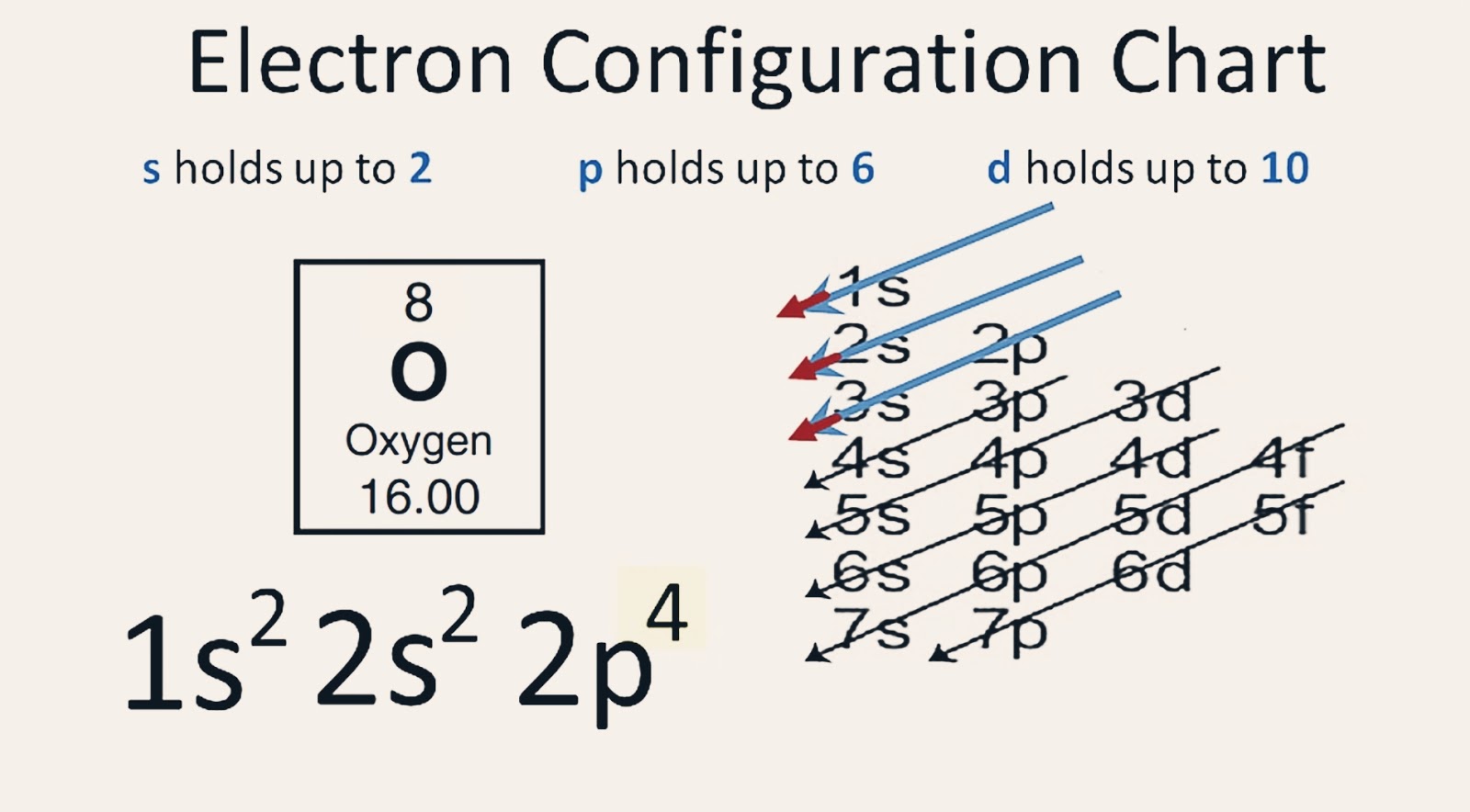
The electron configuration of Oxygen (O) will be 1s2 2s2 2p4. Oxygen is a nonmetal gaseous substance that is also known as a p-block element. It has an atomic number of 8 and is placed after nitrogen in the periodic table. It is one of the most electronegative elements with an electronegativity value of 3.44.

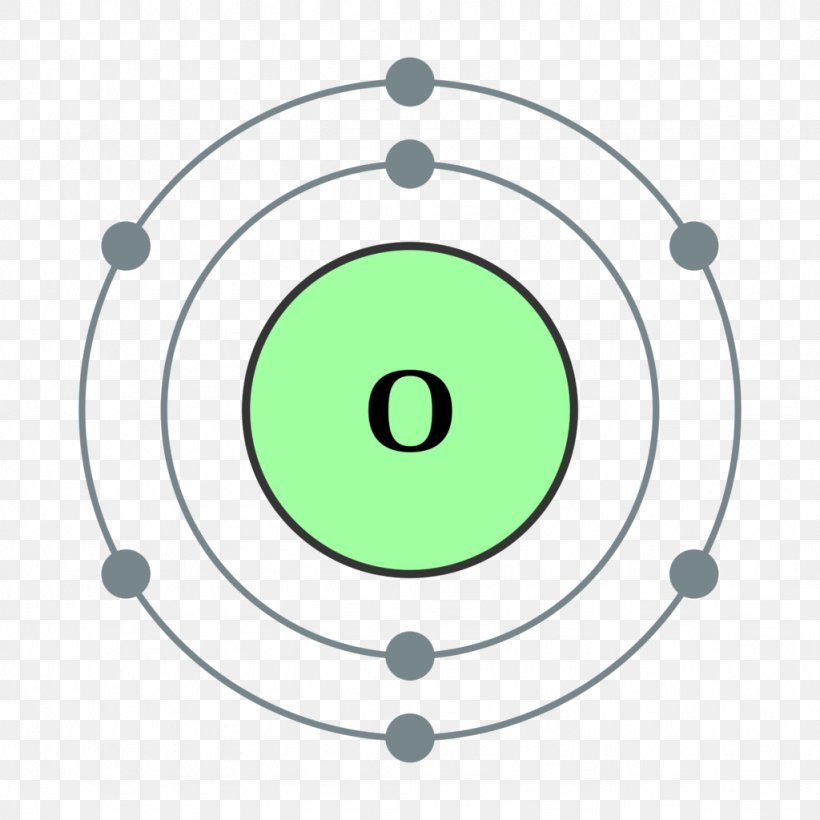
Oxygen Bohr Model (Diagram, Steps To Draw) Techiescientist
Electron configurations are a simple way of writing down the locations of all of the electrons in an atom. As we know, the positively-charged protons in the nucleus of an atom tend to attract negatively-charged electrons.
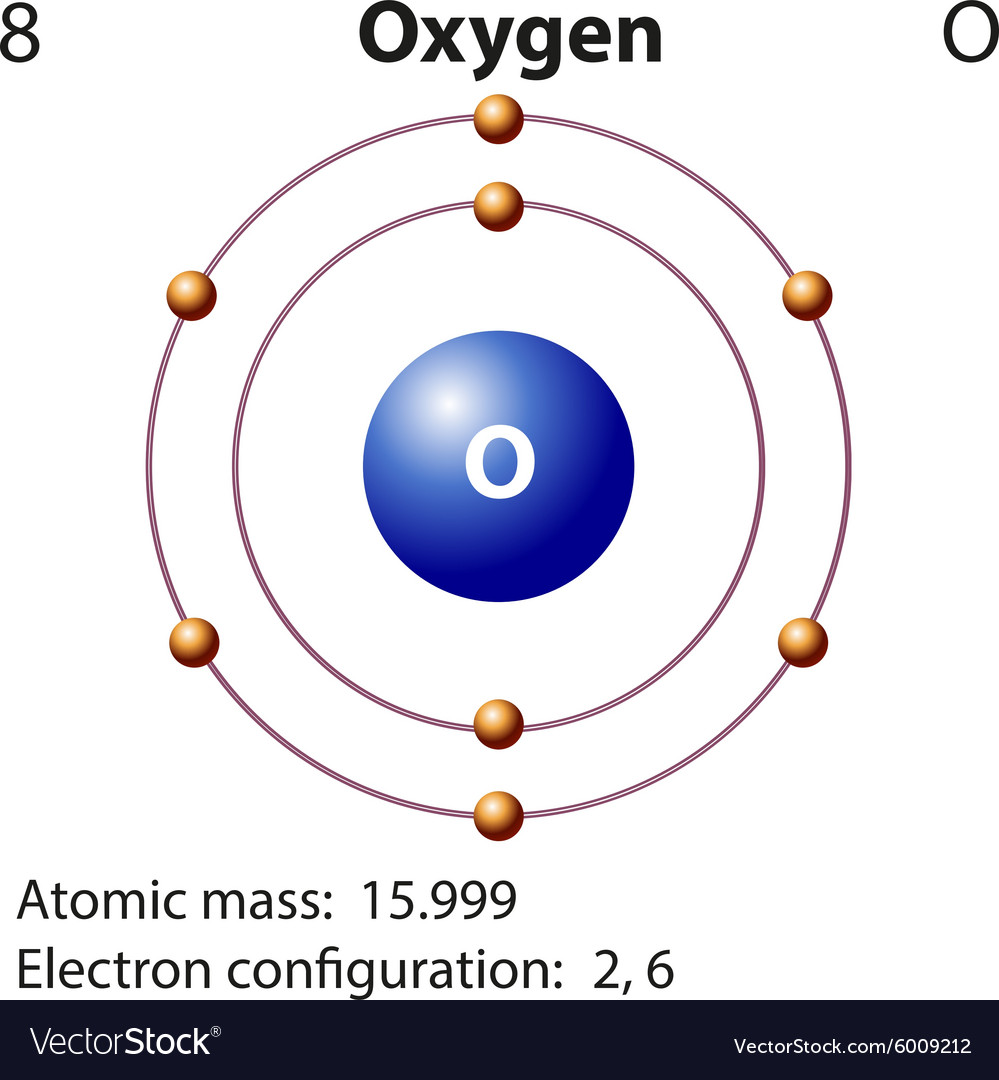
Diagram representation element oxygen Royalty Free Vector
Members of a group typically have similar properties and electron configurations in their outer shell. Period A horizontal row in the periodic table. The atomic number of each element increases by one, reading from left to right.. Oxygen accounts for about 23% of the atmosphere's mass with pairs of oxygen atoms stuck together to make.

Symbol and electron diagram for Oxygen Royalty Free Vector
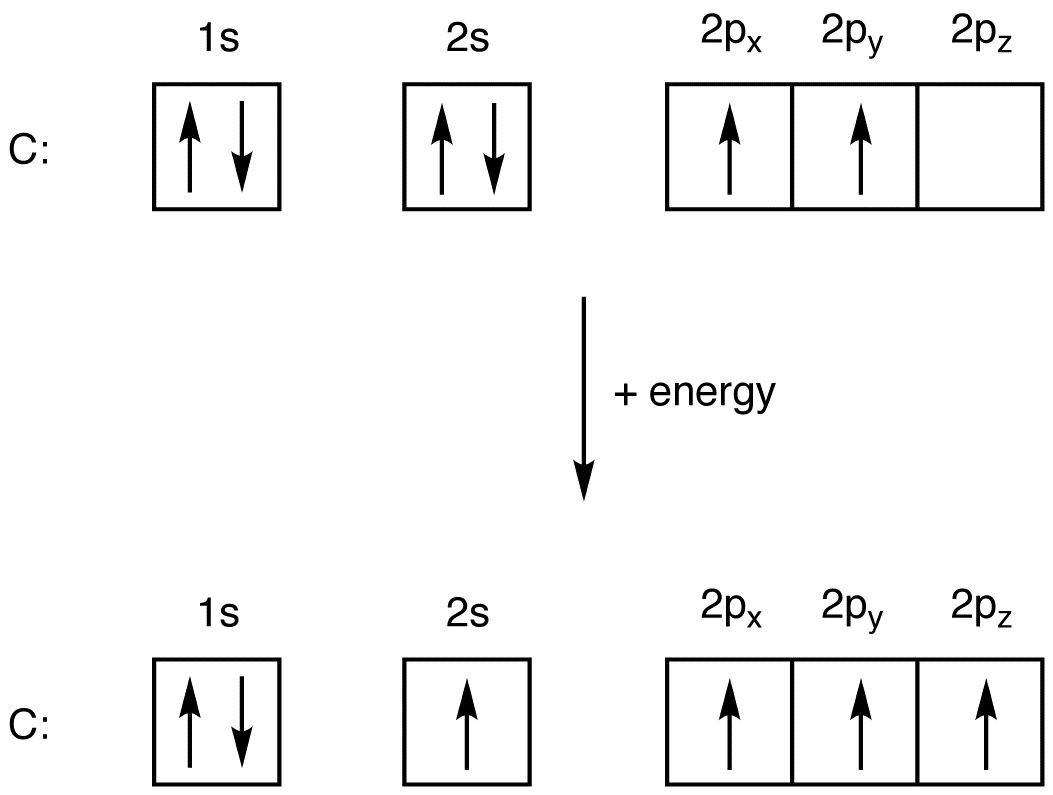
Oxygen is the eighth element with a total of 8 electrons. In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital.
How to Find the Valence Electrons for Oxygen (O)?
We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted 419522 6 months ago At 4:26 , Sal says "In most cases, your valence electrons are going to be your outermost electrons."

Electron configuration of oxygen ion Lousiana
The arrangement of electrons in oxygen in specific rules in different orbits and orbitals is called the electron configuration of oxygen. The electron configuration of oxygen is [ He] 2s 2 2p 4, if the electron arrangement is through orbitals. Electron configuration can be done in two ways. Electron configuration through orbit (Bohr principle)
What is the Electron Configuration of Oxygen Archives Dynamic
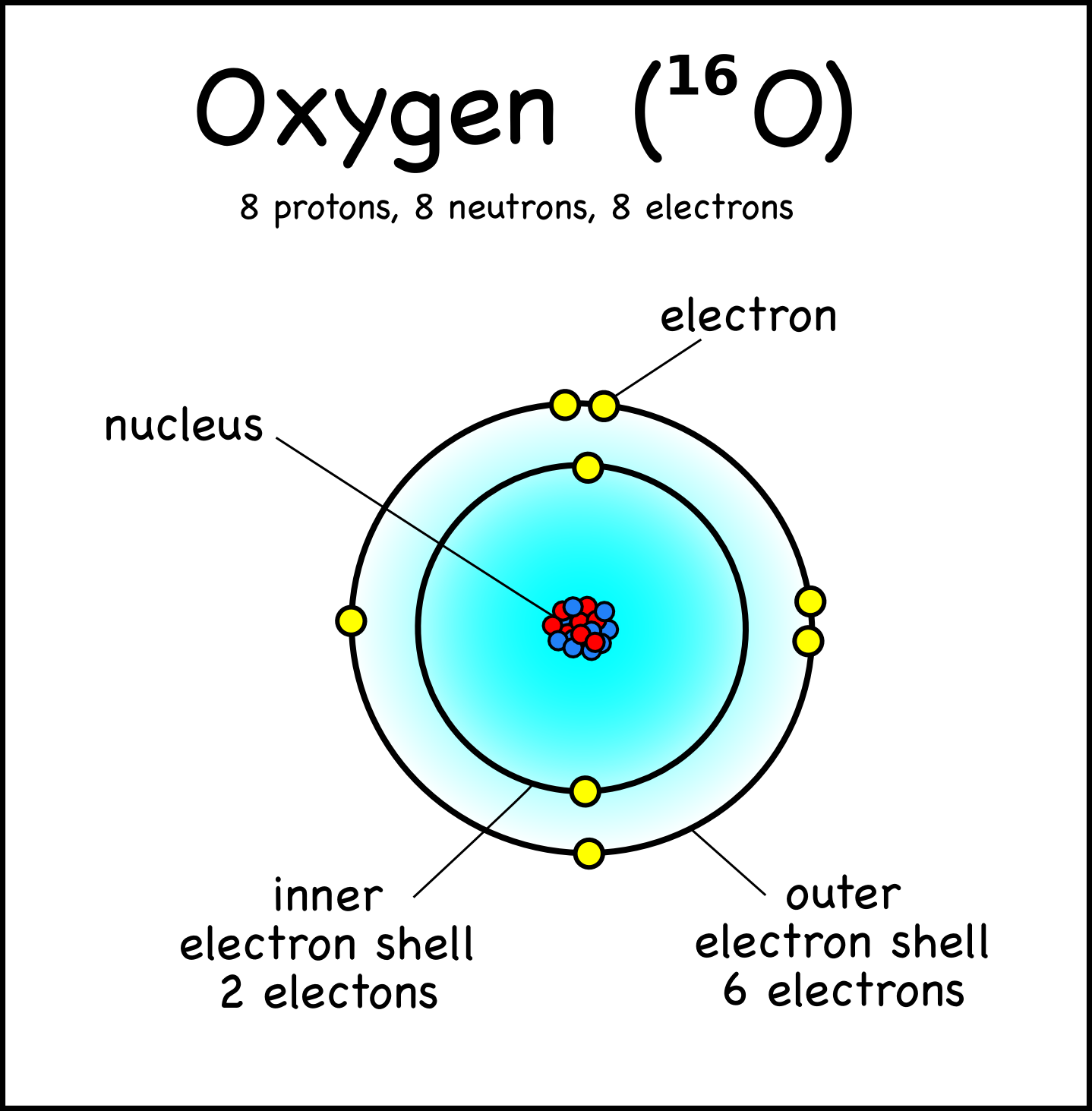
Oxygen Electron Configuration: O is an odourless, colorless, reactive gas. Its atomic number is 8 and it the life-supporting component of the air. It forms almost 20 percent of the earth's atmosphere. It is also the most abundant element in the earth's crust which is found mainly in the form of silicates, oxides, and carbonates.

Isotopes oxygen structure atome labeled Royalty Free Vector
Example 1.6. 3: Carbon and Oxygen. Consider the electron configuration for carbon atoms: 1s 2 2s 2 2p 2: The two 2s electrons will occupy the same orbital, whereas the two 2p electrons will be in different orbital (and aligned the same direction) in accordance with Hund's rule. Consider also the electron configuration of oxygen.
Oxygen Electron Configuration (O) with Orbital Diagram
The electronic configuration of oxygen is- 1s^2 2s^2 2p^4 Note:- For writing the electronic configuration of elements, the Aufbau Principle is used. In Aufbau Principle, the electrons are filled according to the increasing energy level of orbitals. According to the Aufbau Principle, first the atomic number of element is determined (like here oxygen has atomic number 8) and then the electrons.

Electronic configuration of the oxygen atom Download Scientific Diagram
In order to write the O electron configuration we first need to know t.
Atomic Number Oxygen Bohr Model Chemical Element, PNG, 1024x1024px
The arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. We describe an electron configuration with a symbol that contains three pieces of information ( Figure 6.25 ): The number of the principal quantum shell, n,

Electron Configuration Of Oxygen In Ground State
Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen's electron configuration would be O 1s 2 2s 2 2p 4. Special Cases. Configurations of ions present a special case of electron configuration and also demonstrate the reason for the formation of those ions in the first place.

Electron Configuration for Oxygen (O, O2 ion)
Electronic configuration: The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its electronic configuration. Electronic configuration of Oxygen: The electronic configuration of Oxygen is 1 s 2 2 s 2 2 p 4. Oxygen requires two electrons to attain noble gas configuration. Suggest Corrections 24

oxygen atom Chuba Oyolu's Portfolio
In this case, 2+2+6+2+6+2+10+6+2+1= 39 and Z=39, so the answer is correct. A slightly more complicated example is the electron configuration of bismuth (symbolized Bi, with Z = 83). The periodic table gives the following electron configuration: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p65s2 4d10 5p6 6s2 4f14 5d10 6p3.
atoms and molecules Page 3 Montessori Muddle
O 2- Electron Configuration (Oxide Ion) Wayne Breslyn 728K subscribers Join Subscribe Subscribed 799 130K views 4 years ago In this video we will write the electron configuration for O 2-, the.
What Is the Oxygen Electron Configuration(O)?
Let's find the electron configuration of Oxygen! A single oxygen atom has 8 protons and 8 electrons, but how do we know where Oxygen puts its electrons, in w.